The Statement
A video shared on social media claims the federal government is trying to hide reports of adverse reactions to COVID-19 vaccines. The video also makes a number of claims about the meaning of data published by a government-funded organisation that monitors vaccine safety.
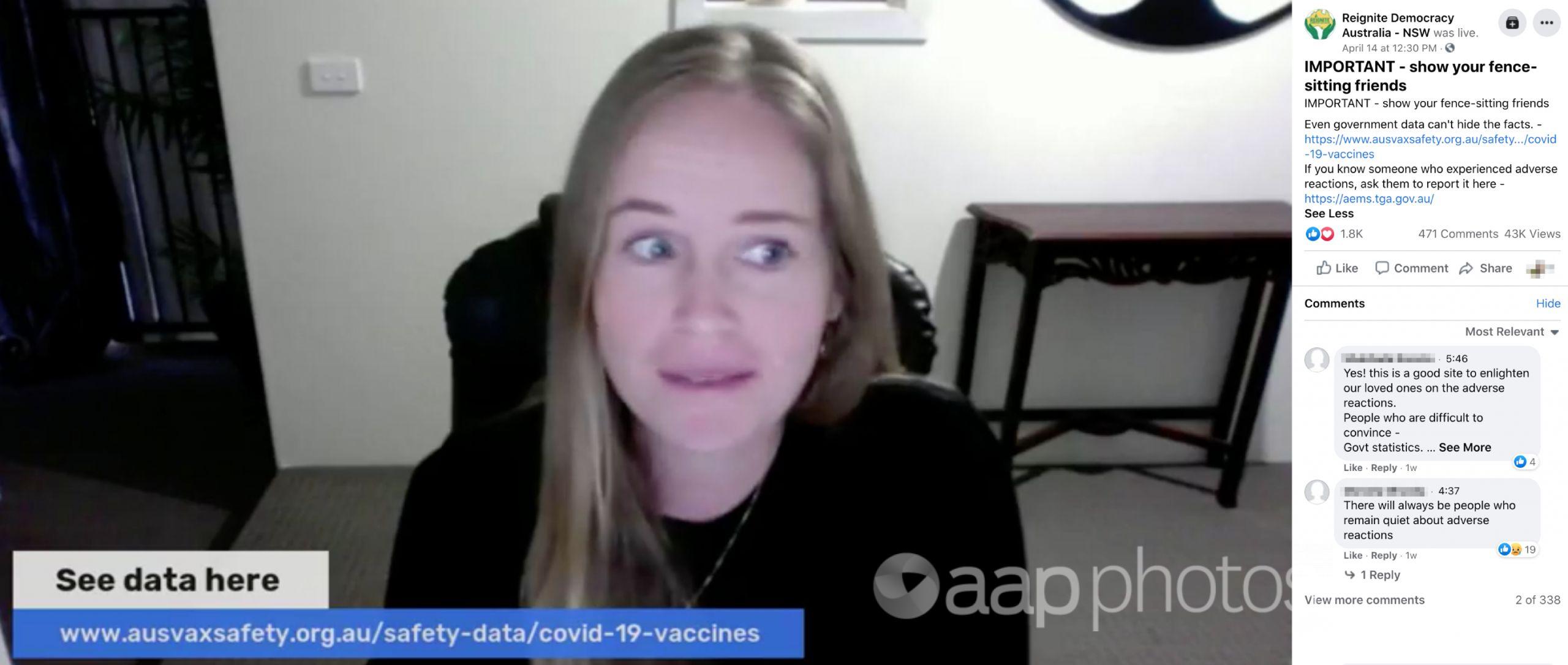
The video was shared to Facebook on April 14 by multiple pages linked to Reignite Democracy Australia, which describes itself as an “advocacy group” and “alternative to the mainstream media”. In the video, the group’s founder, Monica Smit, claims the federal government is “trying to hide adverse reactions” from COVID-19 vaccines.
The video features information published on the website of AusVaxSafety, an organisation which monitors adverse events following immunisation. Ms Smit cites data from the website as evidence that the adverse reaction rate to COVID-19 vaccination is “not a good sign for something that is completely safe” and that 1.6 to 1.8 per cent of people “have ended up in hospital”.
At the time of writing, two posts from the group featuring the video had been shared more than 2100 times and viewed more than 50,000 times.
The Analysis
While the video claims the Australian government is “trying to hide adverse reactions” to COVID-19 vaccines, the data used to support other claims included in the segment is based on published information from a government-funded organisation.
In addition to the work of that entity, AusVaxSafety, government agency the Therapeutic Goods Administration (TGA) also releases a weekly safety report on COVID-19 vaccines as part of its remit of monitoring vaccine safety.
The TGA has also reported publicly in detail on cases where blood clotting and a death were potentially linked to the AstraZeneca vaccine.
In the video post, Ms Smit refers to the AusVaxSafety COVID-19 Vaccine Safety Surveillance page. Archived versions of the page show the data on reported adverse reactions has been published since the beginning of Australia’s vaccine rollout, while the TGA’s reports begin at a similar date.
The page visible in the video shows data based on voluntary survey responses from people who have received the Pfizer Comirnaty vaccine as of April 11. According to the data, 63.3 per cent of 84,439 people reported no adverse event from their first dose and 36.7 per cent reported an adverse event.
AusVaxSafety gathers data via text message or email survey questions sent to people who have received vaccinations from certain providers. Responses are voluntary, and the organisation repeatedly warns on its website against assuming the self-reported events, which were not clinically verified, are all causally related to the vaccine.
Commenting on the data in the video, Ms Smit says (1:43 mark): “36 per cent of people reported some sort of adverse event from the first dose. That’s 36 per cent of people that had some sort of reaction. Now it could have been minor, could have just felt like fatigue and so forth, but still that’s not a good sign for something that is completely safe as (federal health minister) Greg Hunt says”.
However, the types of reported reaction are clearly outlined. A graph on the page shows frequently occurring events, including “injection site pain” the most common at 29.9 per cent, “fatigue” at 19.3 per cent and “headache” at 14.5 per cent.
These are acknowledged on the page as symptoms that are “generally mild and short-lived” and known to occur after vaccination.
“The profile of reported events from AusVaxSafety surveillance for both Comirnaty and COVID-19 Vaccine AstraZeneca is similar to that reported in clinical trials and from post-marketing surveillance overseas, ” an AusVaxSafety spokeswoman told AAP FactCheck in an emailed statement.
“These expected adverse events are related to the immune response to vaccination and are expected to resolve within 1-3 days after vaccination.”
Data from a large-scale clinical trial of the Pfizer vaccine showed 27 per cent of recipients reported an adverse event, although some of these were deemed unrelated to the vaccine. Only four serious adverse events related to the vaccine were reported among more than 20,000 recipients.
AusVaxSafety’s spokeswoman also told AAP FactCheck that the published date only represents information from vaccine recipients who responded to the survey.
“Approximately 30 per cent of vaccinated people who were sent a survey did not complete the survey,” she said.
“The data provides some indication of the rate of events commonly occurring amongst people who have received vaccines, although rates of adverse events may not be a precise estimate of the true rate of adverse events.”
The TGA website states: “AusVaxSafety findings support the TGA’s assessment that the COVID-19 vaccines used in Australia meet safety standards.”
The video contains other statements that misrepresent information on the AusVaxSafety web page. At the 2:13 mark, Ms Smit says that the number of reports on the AusVaxSafety site went up by 13,000 overnight, saying: “This number was 31,000 yesterday, it’s now up to 44,000 in one day”. However text at the top of the AusVaxSafety page, under the “COVID-19 vaccine safety surveillance” headline, clearly states that data is updated weekly.
Referring to a statistic that 21.9 per cent of respondents reported missing work, study or other duties for a short period after a second vaccine dose, Ms Smit says (2:40 mark) that “almost 22 per cent of people have had to miss a day of work or study or some of their duties”.
However, the description beneath that statistic says it actually represents those who missed work, study or duties “for a short period”, adding that the majority of that cohort missed less than one day.
The video also includes a claim (3:30 mark) that 1.6 per cent of AstraZeneca first dose recipients and 1.8 per cent of Pfizer second dose recipients “ended up in hospital”. However the data referred to states that 1.6 per cent of AstraZeneca dose recipients and 1.8 per cent of Pfizer recipients “reported seeing a doctor or going to emergency department in the days after vaccination”. There is no indication as to whether any of the cases were admitted to hospital.
Associate Professor Paul Griffin, an infectious diseases physician and microbiologist from the University of Queensland, reiterated to AAP FactCheck in a phone interview that while the AusVaxSafety system is useful to alert experts of any “safety signals” relating to vaccines, reported symptoms may be unrelated to the vaccine itself. He added that there is also a risk of biases in self-reporting systems.
“We do know that the more significant adverse events are more likely to be reported, particularly if they require medical intervention, for example,” he said.
AAP FactCheck has previously debunked claims regarding the misinterpretation of COVID-19 vaccine adverse events in overseas surveillance systems.
The Verdict
The video makes the false claim that the government is hiding information about COVID-19 vaccine adverse reactions. In fact, the government-funded AusVaxSafety and the TGA, which is part of the federal Department of Health, regularly publish information on reported adverse events.
While the video goes on to refer to real data published by AusVaxSafety, it makes a number of incorrect or misleading interpretations of that information to suggest that it shows the vaccines are unsafe.
False – Content that has no basis in fact.
* AAP FactCheck is an accredited member of the International Fact-Checking Network. To keep up with our latest fact checks, follow us on Facebook and Twitter.
All information, text and images included on the AAP Websites is for personal use only and may not be re-written, copied, re-sold or re-distributed, framed, linked, shared onto social media or otherwise used whether for compensation of any kind or not, unless you have the prior written permission of AAP. For more information, please refer to our standard terms and conditions.