The Statement
A Facebook post has seized on a briefing document listing potential side-effects of COVID-19 vaccines that US authorities would actively monitor, drawing attention to the word “deaths” .
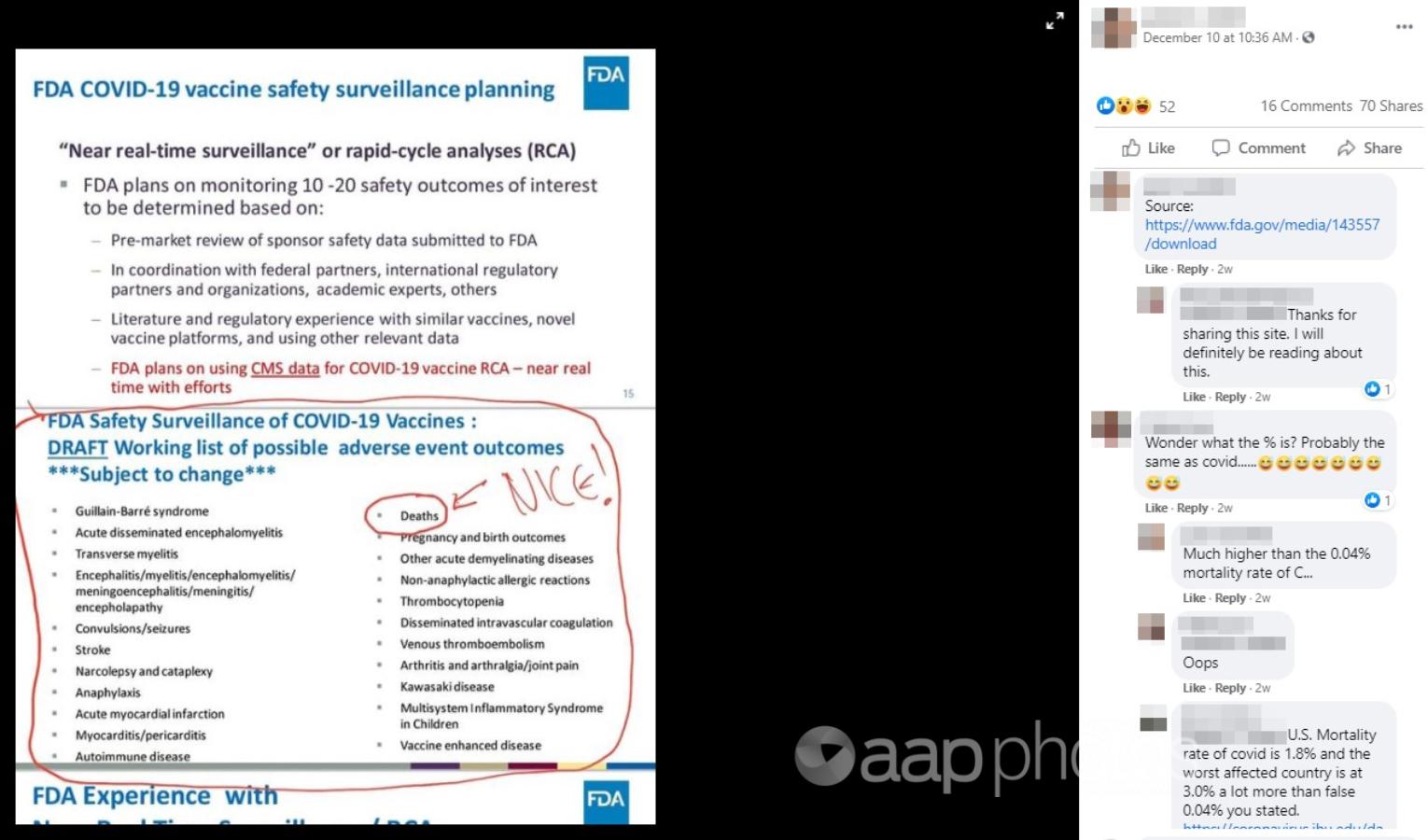
The December 10 post, by a Facebook user listed as living in Christchurch, New Zealand, features an edited image bearing the US Food and Drug Administration (FDA) logo and referring to vaccine safety monitoring.
One section of text in the image, headlined “FDA COVID-19 vaccine safety surveillance planning”, states the “FDA plans on monitoring 10-20 safety outcomes of interest” based on several sources of information.
The following section of text, which has had a red line drawn around it, is headlined: “FDA Safety Surveillance of COVID-19 Vaccines: DRAFT Working list of possible adverse event outcomes ***Subject to change***”
The 22 dot points below include conditions such as stroke, anaphylaxis and Kawasaki disease. It also includes the word “deaths”, which has been circled in red. The comment “NICE!” has been added with an arrow pointing to the circled word.
In the comments on the post, one user has written, “Wonder what the % is? Probably the same as covid…”, to which the post author replied: “Much higher than the 0.04% mortality rate of C…”
At the time of writing, the December 10 post had been shared 70 times and attracted more than 65 reactions and comments.
The Analysis
With the US suffering the world’s highest recorded death toll from COVID-19, the FDA issued an emergency-use authorisation (EUA) for the Pfizer-BioNTech vaccine on December 11 to combat the virus.
In a statement accompanying the announcement, FDA commissioner Stephen M. Hahn described the EUA as “a significant milestone in battling this devastating pandemic that has affected so many families in the United States and around the world”.
Nevertheless, polls show many Americans are reluctant to take a COVID-19 vaccine, with the possibility of side-effects due to the speed at which treatments have been developed cited as a primary concern.
The Facebook post image draws attention to the inclusion of “deaths” in the list of “possible adverse event outcomes”.
The source of the original text featured in the image, minus the drawn-in elements, is an FDA advisory committee meeting in late October (pg 15-16). An FDA spokeswoman confirmed to AAP FactCheck via email that the source was an official briefing document for the October 22 meeting.
However, the spokeswoman added: “No specific vaccine was discussed at that meeting, but rather that meeting was held to discuss – in general – the criteria FDA may take into account when making a decision about emergency use authorisation for a COVID-19 vaccine.
“The list of adverse events on slide 16 is a list of possible outcomes that the FDA may potentially monitor for when conducting safety surveillance. This slide was not referring to a particular vaccine candidate.”
Neither of the FDA’s fact sheets on the Pfizer-BioNtech vaccine for patients or health care providers mention death among its possible side effects.
The only items on the FDA’s “working list”, as featured in the Facebook post, that are included in the possible side effects are joint pain and allergic reactions, although the fact sheet for patients notes “serious and unexpected side effects may occur” from taking the vaccine and it was still being studied.
A fact check by The Associated Press (AP) after the Pfizer-BioNTech vaccine received EUA approval found that social media posts claiming the FDA was intentionally burying the vaccine’s possible “life-threatening” side effects had misrepresented the same slide.
The Pfizer-BioNTech has also been falsely connected to deaths that occurred during its phase three trial, as previously explained by AAP FactCheck.
A week after issuing the first approval, the FDA issued an EUA for Moderna’s COVID-19 vaccine. Fact sheets on the vaccine (see here and here) provide similar side effect warnings to those for the Pfizer-BioNTech product. They do not mention death as a possible outcome.
The Facebook post’s author also suggests in the comments that the mortality rate for COVID-19 is 0.04%, however previous AAP FactCheck investigations have shown the death rate among those infected with the coronavirus is likely more than 10 times this figure (see here, here and here).
The Verdict
The Facebook post has the potential to mislead as it emphasises “deaths” among possible COVID-19 vaccine side effects, while the author also falsely states in the comments that the death rate from the vaccine may be higher than the death rate from the virus.
The FDA confirmed to AAP FactCheck that its list of “adverse event outcomes” included all possible side effects it may monitor for when conducting safety surveillance on vaccines, and did not relate to any specific COVID-19 vaccine.
With the exceptions of potential joint pain and allergic reactions, none of the side effects on the list are among those in the FDA’s official advice on its approved COVID-19 vaccines.
Missing Context – Content that may mislead without additional context.
AAP FactCheck is an accredited member of the International Fact-Checking Network. If you would like to support our independent, fact-based journalism, you can make a contribution to AAP here.
All information, text and images included on the AAP Websites is for personal use only and may not be re-written, copied, re-sold or re-distributed, framed, linked, shared onto social media or otherwise used whether for compensation of any kind or not, unless you have the prior written permission of AAP. For more information, please refer to our standard terms and conditions.