The Statement
A social media post claims more than half of participants across various age ranges in Moderna’s first COVID-19 vaccine trial had adverse reactions – despite the recipients purportedly representing an “extraordinarily” healthy subset of the population.
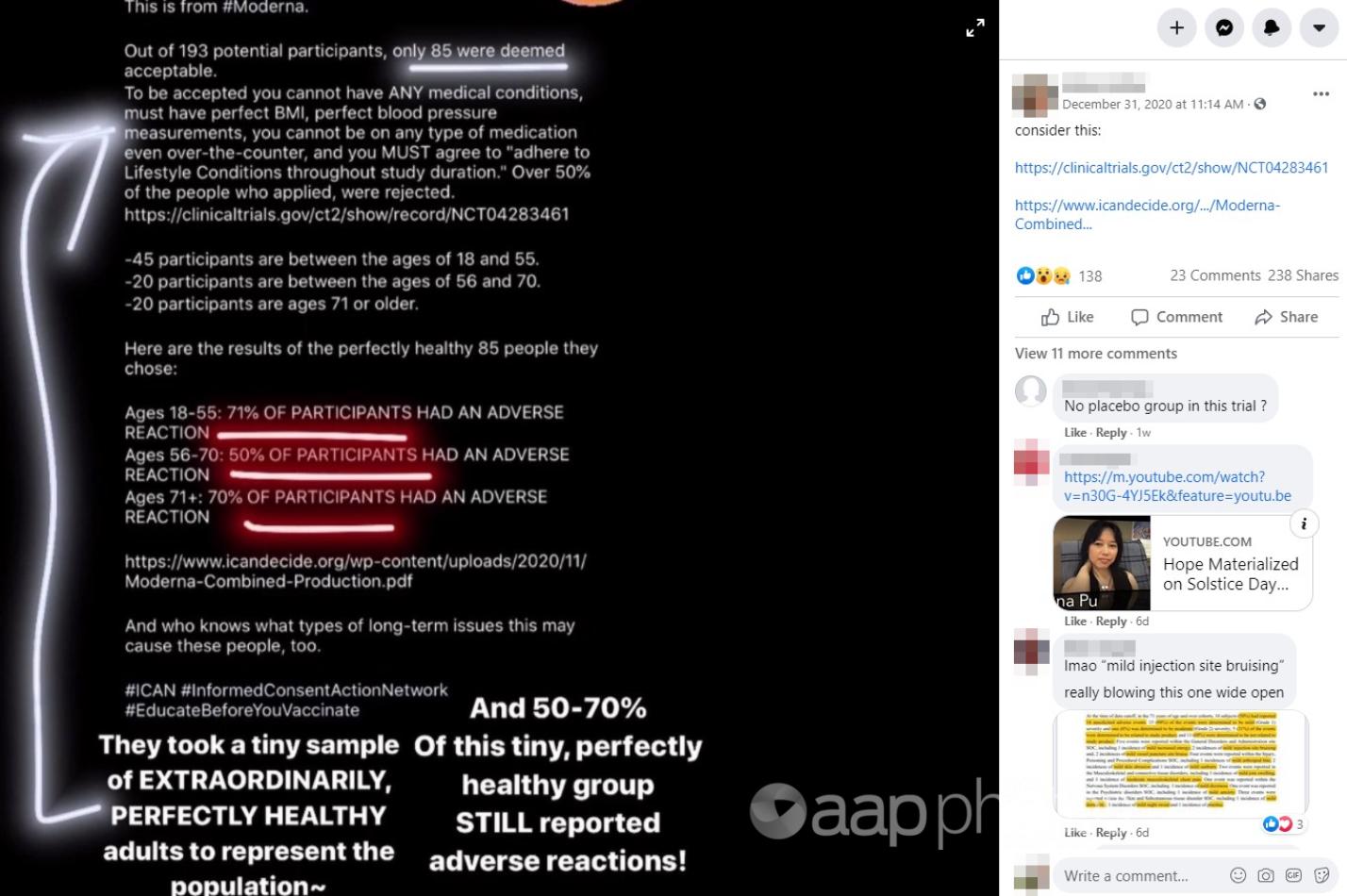
The Facebook post from December 31, 2020, shared by New Zealand users, claims in its image text that Moderna only accepted 85 participants after imposing strict conditions, including that they “cannot have ANY medical conditions, must have perfect BMI, perfect blood pressure, cannot be on any type of medication and MUST adhere to lifestyle conditions”.
The text then divides the participants into age ranges before quoting a series of percentages of the groups who were supposed to have experienced adverse reactions, before adding: “And who knows what types of long-term issues this may cause these people, too.”
“They took a tiny sample of EXTRAORDINARILY, PERFECTLY HEALTHY adults to represent the population ~ And 50-70% of this tiny, perfectly group STILL reported adverse reactions.”
At the time of writing, the post has been viewed more than 17,000 times and has attracted more than 230 shares and 130 reactions.
The Analysis
While many of the participants in Moderna’s COVID-19 vaccine phase one trial experienced adverse reactions, most were minor – and no serious side effects were linked to the treatment.
Moderna’s later phase three trial, used to assess the safety of the vaccine before it was approved for emergency use, involved more than 30,000 participants.
In that trial, fewer than 0.1 per cent of vaccine recipients experienced serious adverse events deemed related to the treatment, a similar rate to that recorded in the placebo group (page 34, table 20).
The post also misrepresents the health status of the people who were selected for the phase one trial, which required participants to be in “good health” but could otherwise be overweight with some existing medical conditions.
The US Food and Drug Administration (FDA) issued an emergency use authorisation (EUA) for Moderna’s COVID-19 vaccine on December 18, 2020, making it the second COVID vaccine to be approved in the country after the Pfizer-BioNTech vaccine.
Moderna began working on a potential COVID-19 cure in January 2020, co-operating with the US National Institutes of Health (NIH) in the phase one vaccine trials which began in March 2020.
According to Australia’s National Health and Medical Research Council, phase one clinical trials are done to “test a new biomedical intervention for the first time in a small group of people (e.g. 20-80) to evaluate safety (e.g. to determine a safe dosage range and identify side effects)” before moving onto large trial groups in the later phases.
As the source of its information, the Facebook post included links to the phase one clinical trial listing from February 25 and a purported “safety summary report” prepared by the Statistical and Data Coordinating Center (SDCC) Emmes and dated June 2.
The report was published by the US-based anti-vaccine group Informed Consent Action Network (ICAN). AAP FactCheck was unable to verify the authenticity of the report, however an ICAN spokeswoman said it was produced in response to a lawsuit against the National Institutes of Health (NIH).
A NIH spokeswoman said: “Due to the length and complexity of the report posted on the ICAN website, NIAID is unable to verify its legitimacy.” Moderna did not respond to a request for comment, while Emmes referred inquiries to the NIH.
However, preliminary reports on the safety and efficacy of the vaccine have been released for 85 trial participants across two groups, those aged from 18 to 55 and those aged 56 and older.
Neither trial resulted in any serious adverse events linked to the vaccinations, according to the preliminary reports, although mild symptoms such as headaches, muscle pain and localised pain at the injection site were common.
The post claims that participants in the phase one trial represented “extraordinarily, perfectly healthy adults” with “perfect” body mass index (BMI) and blood pressure readings.
However, eligibility criteria for the trial showed applicants only needed to be in good health with a BMI of up to 35 in the younger group, or 30 in the older cohort. Both are classified as obese.
The average BMI of participants in both groups was around 25, classed at the bottom end of the “overweight” range (see table 1 in the reports for 18 to 55 and 56 and older).
To be eligible for the trial, candidates also needed to have systolic blood pressure of no more than 150 mm Hg. Anyone with a systolic reading above 130 is considered to be in the stages of hypertension.
The American Heart Foundation says people with hypertension are likely to be prescribed lifestyle changes and possible blood-pressure medication.
Those with “significant” medical or psychiatric conditions – such as heart disease or immunodeficiency – were barred from the trial, but participants could have other acute or chronic conditions as long as they were deemed unlikely to risk the person’s safety during the treatment period.
Dr Phillip Reece, a senior fellow in the Department of Pharmacology and Therapeutics at the University of Melbourne, told AAP FactCheck entry criteria for phase one trials were intended to be restrictive.
“As the initial Phase I trial is the first trial in humans, only healthy subjects are recruited to avoid the risk of harm to those with other medical conditions. Volunteers can vary significantly in their medical status at screening, so it is not surprising if a large number do not meet the entry criteria,” Dr Reece said in an email.
The Facebook post highlights the number of adverse reactions experienced by the “perfectly healthy group” in the trial to cast doubt on the safety of the vaccine.
However, additional information in the unverified safety summary report, which lists every purported reaction experienced during the phase one trial, states the majority of reactions were mild and not caused by the vaccine itself.
Among the 18-55 age bracket, for example, it stated 32 of the 45 participants (71 per cent) experienced 82 unsolicited adverse reactions. Of these, 50 were deemed unrelated to the vaccine, while 64 were classed as mild.
Examples of adverse events included in that tally, according to the report cited in the post, but not necessarily linked to the treatment include everything from “mild sunburn” and “mild arthropod bite” to flatulence, decreased appetite, hypertension, increased energy and nasal congestion.
Supplementary data included with the published preliminary report notes 90 unsolicited adverse events among the 18-55 group, none of which were serious. The majority were mild and unrelated to the vaccine.
Nikolai Petrovsky, a professor of medicine at Flinders University in Adelaide, told AAP FactCheck in an email that minor adverse reactions are extremely common in vaccine trials.
“In total, having more than half of the participants in the vaccine trial report mild and transient adverse events is completely normal and to be expected,” Prof Petrovsky said.
“When a regulator looks at such data, they are not interested in the total number of adverse events which are mainly mild and transient but instead will focus on the incidence of serious adverse events, particularly those that could potentially be life threatening.”
Associate Professor Paul Griffin, an infectious diseases physician and microbiologist at the University of Queensland, told AAP FactCheck that a large number of adverse events – particularly if they were severe and related to the vaccine – would lead to the phase one trial being discontinued, and the vaccine wouldn’t have proceeded to the phase three stage.
The FDA’s EUA review of the Moderna vaccine’s phase three trial said that adverse events were reported in a higher proportion of vaccine recipients than placebo recipients due to “reactogenicity” – or the expected inflammatory response soon after vaccination (page 33).
The review said there was no difference in serious adverse events between the vaccine and placebo groups. Four people died during the trial period in both groups, however none of the deaths were deemed related to the treatments.
“If the related events are similar to what is seen in the placebo group, then that means there’s not a significant issue with the actual vaccine itself,” Prof Griffin added.
The Verdict
The post falsely claims that Moderna’s COVID-19 vaccine phase one trial only involved “extraordinarily” healthy participants with perfect BMI and blood pressure readings, when in fact candidates could be overweight and experiencing hypertension along with other medical conditions.
It also quotes figures on adverse reactions during the trial that is missing important context. While participants did experience a range of adverse reactions, the majority of those reactions were mild – and no serious events were linked to the vaccine.
According to experts from the University of Melbourne, University of Queensland and Flinders University, it is expected that mild adverse reactions would be reported in phase one trials.
Partly False – Content that has some factual inaccuracies.
Updated Tuesday, January 19, 2020 17.23 AEDT: Comment from ICAN included.
* AAP FactCheck is an accredited member of the International Fact-Checking Network. If you would like to support our independent, fact-based journalism, you can make a contribution to AAP here.
All information, text and images included on the AAP Websites is for personal use only and may not be re-written, copied, re-sold or re-distributed, framed, linked, shared onto social media or otherwise used whether for compensation of any kind or not, unless you have the prior written permission of AAP. For more information, please refer to our standard terms and conditions.