The Statement
Social media posts claim an ingredient contained in Moderna’s COVID-19 vaccine has been deemed unsafe for human use.
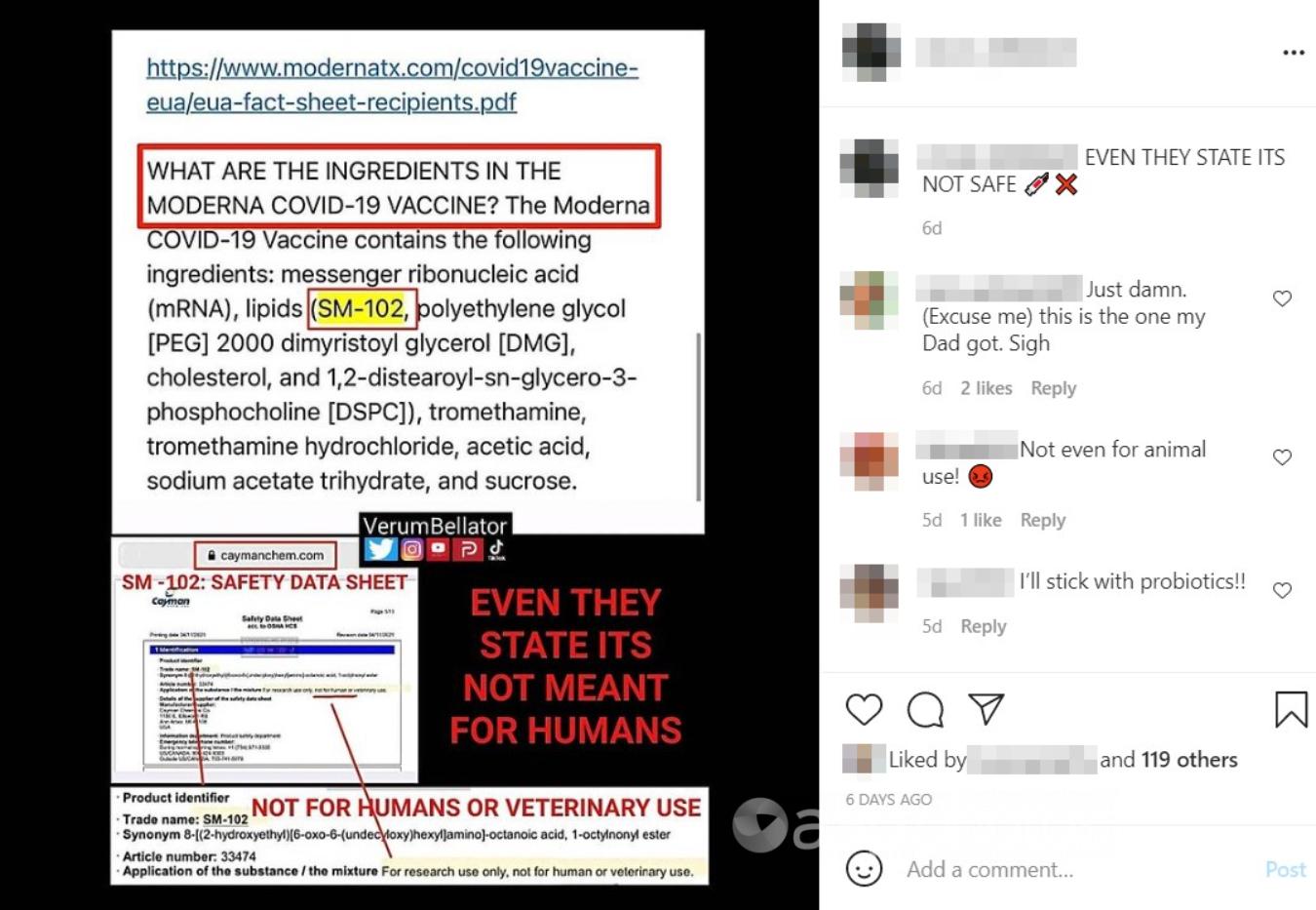
One Instagram post, from May 18, features a list of the vaccine’s ingredients with the term “SM-102” highlighted.
It also includes a screenshot of a “safety data sheet” from Cayman Chemical for a product identified by the trade name SM-102. The data sheet includes the words: “For research use only, not for human or veterinary use.”
Red text has been added to the post that reads: “Even they state its (sic) not meant for humans.”
Various versions of the same claim have been shared online, including on Facebook and elsewhere on Instagram. The claim was also promoted by US conspiracy theorist Alex Jones.
The Analysis
Despite the post’s claim, Moderna’s COVID-19 vaccine is not unsafe for humans. The Cayman Chemical solution highlighted in the post contains a toxic additive not included in the Moderna treatment, and it is this component that makes it potentially dangerous to people and animals.
In comparison, the Moderna vaccine has been found to be safe and effective for human use.
The Australian government announced on May 13 it had secured 25 million doses of the Moderna mRNA vaccine to bolster the country’s stock of COVID-19 inoculations. Supply to the public will only begin if the Therapeutic Goods Administration (TGA) approves it for use.
Since the announcement, several social media users in Australia have circulated claims that one ingredient, SM-102, is dangerous to recipients based on a safety data sheet from a biotechnology company Cayman Chemical.
However, the document details two components in the mixture (page 3). Under “dangerous components” it lists chloroform, which was once used as an inhaled anaesthetic for surgery but is now mainly used as an industrial solvent.
This makes up 90 per cent of the mixture, while the remaining 10 per cent is SM-102, a type of lipid – or fat – used in the Moderna vaccine. SM-102 is listed under “other ingredients” in the Cayman Chemical safety sheet. Elsewhere, the document makes several other references to toxicology and safety information for the chloroform component only.
Dr Bryan Williams, emeritus director at Melbourne’s Hudson Institute of Medical Research, told AAP FactCheck that SM-102 was a lipid that acted as a coating to protect the mRNA in the vaccine from breaking up when it entered a recipient’s cell.
“The very small amount of SM-102 along with the other lipid components in a vaccine dose would pose no risk to humans as is clear by its approval (by regulatory agencies) for use,” he said.
The Centers for Disease Control and Prevention (CDC) in the US, where the vaccine has been rolled out, states the Moderna vaccine is highly effective at preventing COVID-19 illness in a diverse range of people, including those with underlying medical conditions.
Common side effects include pain in the arm at the injection site, headache, muscle pain and chills, but the CDC says most of these symptoms are mild to moderate in severity.
The European Medicines Agency has authorised the Moderna vaccine for use across the European Union after “thoroughly assessing the data on the quality, safety and efficacy of the vaccine”.
Dr Pall Thordarson, a professor of chemistry from UNSW Sydney with expertise in lipids, nanoparticles and RNA, told AAP FactCheck in an email the claim that Moderna vaccines are unsafe for humans is “bogus”.
Prof Thordarson noted the Cayman Chemical mixture was mainly composed of chloroform and was intended for research purposes.
“The safety data sheet refers to the risk associated with that mixture – where all the risk factors, as far as I can see, are from the chloroform component,” he said.
In comparison, Prof Thordarson said Moderna used pure SM-102. This would be dissolved in pure ethanol, not chloroform, and mixed with the mRNA before the ethanol is filtered out in the final product, he said. Chloroform does not appear among the vaccine’s list of ingredients.
The total amount of SM-102 entering the body after two injections would be well under one milligram, less than the mass of an ant, Prof Thordarson added.
“We are talking very small amounts of a lipid that nothing indicates has any risks associated with it,” he said.
Prof Thordarson likened the misinterpretation of the safety data sheet to someone who “took a look at a strawberry vodka bottle with all the warnings on it and concluded that strawberries are very dangerous, can cause you to drive erratically and even harm an unborn child”.
Cayman Chemical released a statement on May 19 in which it said its SM-102 product contained both chloroform, which had several known hazards, and SM-102, which multiple agencies identified as having no hazards attached.
The Verdict
The presence of the lipid SM-102 in the Moderna COVID-19 vaccine does not mean it is unsafe for human use. The document presented as evidence of this risk relates to a laboratory-grade mixture, mostly made up of chloroform – which is identified in the document as the toxic substance. In comparison, the vaccine uses the lipid but does not contain chloroform.
Multiple medicines agencies have determined the Moderna vaccine is safe and effective for recipients.
False – Content that has no basis in fact.
* AAP FactCheck is an accredited member of the International Fact-Checking Network. To keep up with our latest fact checks, follow us on Facebook and Twitter.
All information, text and images included on the AAP Websites is for personal use only and may not be re-written, copied, re-sold or re-distributed, framed, linked, shared onto social media or otherwise used whether for compensation of any kind or not, unless you have the prior written permission of AAP. For more information, please refer to our standard terms and conditions.

















