As Company Advances Planning for FDA Submission and Future Growth
MINNEAPOLIS, Sept. 19, 2022 /PRNewswire/ — HistoSonics, (www.histosonics.com), the developer and manufacturer of a completely non-invasive platform and proprietary sonic beam therapy, announced today the publication of results from their prospective Phase I clinical trial, named the THERESA Trial, conducted in Barcelona, Spain. The study reported results on eleven targeted tumors in the eight patients treated who all had unresectable end-stage multifocal liver tumors: colorectal liver metastases (CRLM) in 5 patients (7 tumors), breast cancer metastases in 1 (1tumor), cholangiocarcinoma metastases in 1 (2 tumors), and hepatocellular carcinoma (HCC) in 1 (1tumor). Importantly, the primary endpoint of technical success was achieved in all procedures. The secondary safety profile endpoint was achieved as an independent data safety monitoring board identified no device-related adverse events observed up to 30 days after histotripsy.
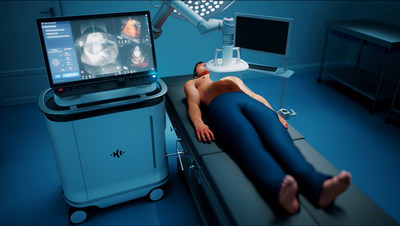
The science of histotripsy uses focused sound energy to produce controlled acoustic cavitation that mechanically destroys and liquifies targeted liver tissue at sub-cellular levels. Uniquely, the HistoSonics platform also provides physicians the ability to plan, target, treat and monitor the destruction of tissue under continuous real-time visualization and control, unlike any modality that exists today. Results of the study, recently published in the International Journal of Hyperthermia (THERESA Trial), demonstrated that the HistoSonics’ histotripsy system met its co-primary endpoints of acute safety (30 day) and technical success (ability to treat targeted volume of liver tissue) in liver tumors of both primary and secondary origin.
“Being able to safely target and treat tumors in this advanced patient population, non-invasively and without ionizing radiation means a great deal not only to patients with multi-focal disease but could also provide a bladeless surgical option for patients in earlier stages”, said co-Principal Investigator Joan Vidal-Jove MD, PhD, a surgical oncologist with the Kuhab Institute in Barcelona. Dr. Vidal-Jove’ and Dr. Xavier Serres led the study at the three Barcelona based hospitals that participated in the trial which included, Vall d’Hebron University Hospital, Mutua Terrassa University Hospital and Clinica Diagonal.
The published THERESA Trial results also demonstrated that the histotripsy system could be used to successfully target and treat multiple tumors in the same procedure, or single fraction, with one patient receiving three treatments to 3 distinct tumors, one patient receiving treatment for 2 tumors and the remaining patients having only one tumor treated. “The publication of the THERESA Trial represents a landmark study demonstrating the safety and acute technical success of hepatic histotripsy, and we look forward to results of our next generation histotripsy system in destroying targeted liver tumors in our ongoing #HOPE4LIVER Trials”, says Dr. Joseph Amaral, HistoSonics’ VP of Clinical and Medical Affairs. HistoSonics intends to use data from the #HOPE4LIVER Trials being conducted the US and Europe to support regulatory submission to the FDA in the coming months.
HistoSonics also commented that they have begun meeting with the FDA on their plans for a kidney tumor trial to commence in 2023 and are also currently conducting pre-clinical translational studies in the pancreas. The Company has also recently completed renovation of its manufacturing facility in Plymouth, MN and has hired additional personnel across all functions to prepare for upcoming growth.
HistoSonics is a privately held medical device company developing a non-invasive platform and proprietary sonic beam therapy utilizing the science of histotripsy, a novel mechanism of action that uses focused ultrasound to mechanically destroy and liquify unwanted tissue and tumors. The company is currently focused on the continued development of its Edison™ Platform, global clinical studies, and new strategic projects including future clinical applications and platforms. HistoSonics has offices in Ann Arbor, Michigan and Minneapolis, MN.
For more information please visit: www.histosonics.com/

SOURCE HistoSonics, Inc.



















