HSINCHU, March 19, 2024 /PRNewswire/ — ICP DAS-BMP dazzled at the prestigious MD&M West Show, where its high-quality medical-grade thermoplastic polyurethane (TPU) garnered orders from top manufacturers in U.S. and Japan. Concurrently, part of the company’s TPU products passed the ISO10993-6 test for local effects after 90-day implantation, positioning them as ideal materials for producing indwelling medical devices intended for use over 30 days.
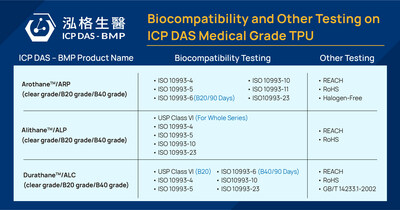
ICP DAS-BMP, Asia’s first medical-grade TPU supplier, produces three main series of products: Alithane™ (ALP series), Durathane™ (ALC series), and Arothane™ (ARP series). The company is set to launch a new Durathane™ (ARC series), known for its exceptional biostability. All the TPU series are available in different hardness levels, colors, and concentrations of radiopaque fillers (Tungsten/Barium Sulfate). These materials are widely utilized in manufacturing cardiovascular, urological, and gastrointestinal devices, as well as medical and electromedical consumables.
ICP DAS-BMP’s Arothane™ ARP-B20 (20% Barium Sulfate) and Durathane™ ALC-B40 (40% Barium Sulfate) passed the ISO10993-6 90-day implantation test. This qualifies them for long-term invasive or implantable medical applications such as peripherally inserted central catheters (PICC) and port-a-cath. The company plans to conduct long-term implantation tests for other specifications of TPU products based on customer demand, while advancing research and development to uphold TPU product quality.
Medical-grade TPU differs from other plastics by emitting minimal VOCs during manufacturing and having low wastewater discharge, making it extremely environmentally friendly. It contains no plasticizers or heavy metals, ensuring excellent biocompatibility and blood compatibility, and enhancing ESG sustainability. ICP DAS-BMP aims to offer safer, stable, and eco-friendly medical-grade TPU, collaborating with global medical device manufacturers to create long-term benefits and value.
Meet the company’s experts at:
CMEF (ICMD) 2024 (April 11-14)
Booth Hall 8.1, M44, M46
NECC (Shanghai), China
Medtec Japan 2024 (April 17-19)
Booth 2022
Tokyo Big Sight East 2~3 Hall, Japan
About ICP DAS-BMP
ICP DAS-BMP, a Taiwan-based TPU manufacturer and supplier certified with ISO 13485, operates specialized laboratories dedicated to quality management. Drawing upon three decades of industrial automation expertise from its parent company ICP DAS, ICP DAS-BMP has implemented smart factory practices to enhance product quality and expedite delivery times. Additionally, the company provides responsive after-sales support and offers flexible solutions for small order quantities.
For more information, please visit: https://bmp.icpdas.com/
For TPU products and inquiries, contact: sales_bmp@icpdas.com
SOURCE ICP DAS Co., Ltd.