An Instagram post claims US authorities approved Pfizer’s updated COVID booster vaccine for the BA.4 and BA.5 Omicron subvariants based only on data from trials on mice.
The pharmaceutical company created the updated vaccine to better target the now dominant Omicron variant.
The claim relates to approval in the US, with the Food and Drug Administration (FDA) giving the vaccine “emergency use authorization” in August.
In Australia, the Therapeutic Goods Administration (TGA) has given provisional determination. Pfizer is now required to submit a market authorisation application before the vaccine is given the all-clear for use in Australia.
The claim was made in a September 5 post (archived here), which included a screenshot of a Science.org article about the updated boosters.
“So Pfizer submitted a request for emergency use authorization for booster #5 and the only data they presented was from trials in mice,” the post’s caption reads.
Various versions of the claim have been shared on Facebook (here, here and here) including by Australian Christian Lobby managing director Martyn Iles, who deleted his post after being fact-checked by AFP.
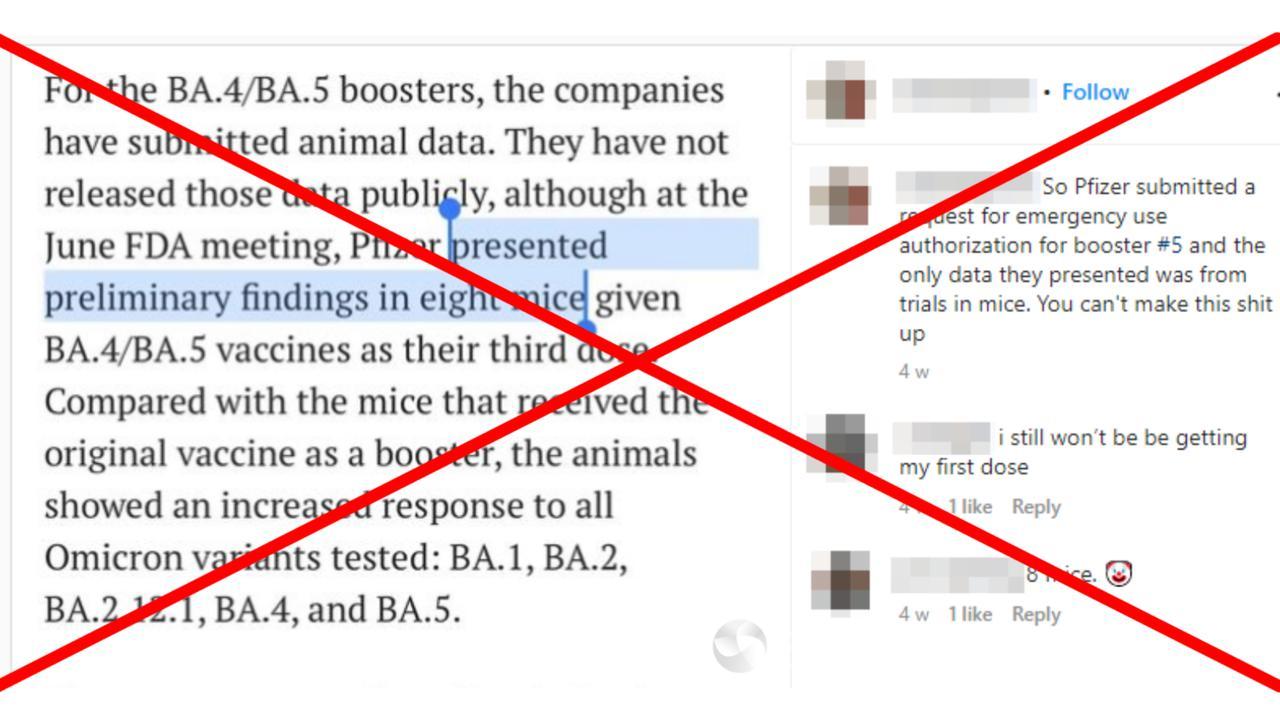
The claims are misleading.
While Pfizer–BioNTech did provide preliminary animal data collected from eight mice, it also submitted data from a human study for a similar booster targeting the BA.1 subvariant and safety and effectiveness data for the original mRNA COVID-19 vaccines.
On August 31, the FDA announced it had granted emergency use authorisation for both Moderna and Pfizer’s updated booster vaccines, which target the subvariants.
The boosters are known as bivalent vaccines because they include two mRNA components of the SARS-CoV-2 virus – one from the original strain and one from BA.4 and BA.5, which have identical spike proteins.
Pfizer told AAP FactCheck its submission included “clinical, pre-clinical and manufacturing data”, with the mice trials being the pre-clinical data.
“To advance the Omicron BA.4/BA.5 bivalent vaccine as rapidly as possible, regulators have advised that our submissions be based on safety and immunogenicity data generated in adults with an Omicron BA.1-adapted bivalent vaccine and supported by BA.4/BA.5 bivalent pre-clinical data and BA.4/BA.5 bivalent chemistry, manufacturing and controls data,” a Pfizer spokeswoman said via email.
“Pre-clinical data showed a booster dose of Pfizer and BioNTech’s Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine generated a strong neutralising antibody response against Omicron BA.1, BA.2, and BA.4/BA.5, as well as the original wild-type virus.”
In a presentation to the FDA in June, Pfizer outlined data relating to both BA.1 and BA.4/5 booster vaccines. This included a clinical study in which 640 participants were given a booster dose of the BA.1 bivalent vaccine (page 11), along with preliminary data from eight mice given two doses of the BA.4/5 bivalent vaccine (page CC-25).
Scott Roberts, an assistant professor and associate medical director of infection prevention at Yale School of Medicine, told AAP FactCheck the preliminary mice data helped support the FDA decision.
“But this was done in conjunction with more human clinical trial data using a BA.1 bivalent booster which showed no new concerning safety signals, so the assumption is this would also hold true for the BA.5 bivalent booster,” Dr Roberts said in an email.
“Unless the vaccine manufacturer changes how the vaccine is produced, they don’t need to perform new and updated clinical trials since the changes were relatively minor (the mRNA was modified to BA.5).”
Andrew Pekosz, a professor in molecular microbiology and immunology at John Hopkins University, also confirmed the BA.4/5 booster was approved based on a number of different parameters, including human data from the BA.1 bivalent vaccine.
“This bivalent booster showed better recognition of various Omicron sublinages and was therefore put forward to the FDA/CDC as an updated booster with better activity,” Prof Pekosz told AAP FactCheck in an email.
“The FDA took this data and – given the millions of doses of mRNA vaccines that showed safety – recommended that the bivalent booster contain what they thought would be the dominant Omicron in the fall, BA.5.
“That prediction turned out to be largely correct so now we have a bivalent booster that actually matches the circulating Omicron strain and boosts immune response from the original vaccine strain.
“This is an ideal situation.”

Dr Roberts said the process of authorisation is similar to what happens with influenza vaccines each year.
“There is not time to perform clinical trials with the new influenza vaccine virus every year (since the results/approval would not occur until many months into the flu season, well past the time point when many would have been exposed to influenza), so a mouse model is used to determine immune response and the new strains are used to update the vaccines,” he said.
“The vaccine is manufactured in the same method so no new clinical trials are needed.”
The Verdict
The claim Pfizer only provided data from mice trials in its application to US authorities for its BA.4/5 COVID-19 booster vaccine is misleading. The authorisation was also based on pre-clinical and clinical data for the original mRNA vaccines and a bivalent BA.1 booster vaccine.
Experts told AAP FactCheck the emergency use authorisation process for the BA.4/5 boosters is similar to what happens with influenza vaccines each year.
Misleading – The claim is accurate in parts but information has also been presented incorrectly, out of context or omitted.
* AAP FactCheck is an accredited member of the International Fact-Checking Network. To keep up with our latest fact checks, follow us on Facebook, Twitter and Instagram.
All information, text and images included on the AAP Websites is for personal use only and may not be re-written, copied, re-sold or re-distributed, framed, linked, shared onto social media or otherwise used whether for compensation of any kind or not, unless you have the prior written permission of AAP. For more information, please refer to our standard terms and conditions.